What are colloids?
Colloids are particles or solids suspended in water that are between 1 nm (the cut-off between dissolved and undissolved particles) and 1-2 micron in diameter. What differentiates colloids from other suspended solids is this 2 micron size cut-off. Due to the small size of colloids they will remain suspended in the water indefinitely while particles larger than 1-2 micron will settle out due to gravity. Colloids stay suspended due to a balance between Brownian motion and a slow settling rate due to gravity.
Where do colloids occur?
Colloidal particles are common in surface water sources, particularly dams and rivers with clay beds. Rivers can have seasonal increases in colloidal particles due to storm surges churning up the river bed. Ash from volcanic eruptions or bushfires can also introduce colloidal particles into surface water sources.
Groundwater sources such as bores can silt up overtime which can introduce a source of colloidal particles. Physical colloids are predominantly aluminosilicates, manganese or aluminium oxide and quartz. Colloidal iron and silica are also commonly found in natural water sources. Natural Organic Matter (NOM) can also produce colloids through the growth of algae or the by-products of bacteria.

What issues do colloids cause?
Multimedia filtration, a common filtration system for water, filters suspended solids out that are larger than 3 micron. However colloidal particles will not be filtered out by multimedia filtration. Any remaining colloids in the water can clog distribution systems and pumps. Another issue caused by colloids is fouling membrane filtration methods particularly nanofiltration or reverse osmosis. As colloids bypass traditional physical filters they will physically clog the pores of reverse osmosis and nanofiltration membranes.
Testing for colloids in your water
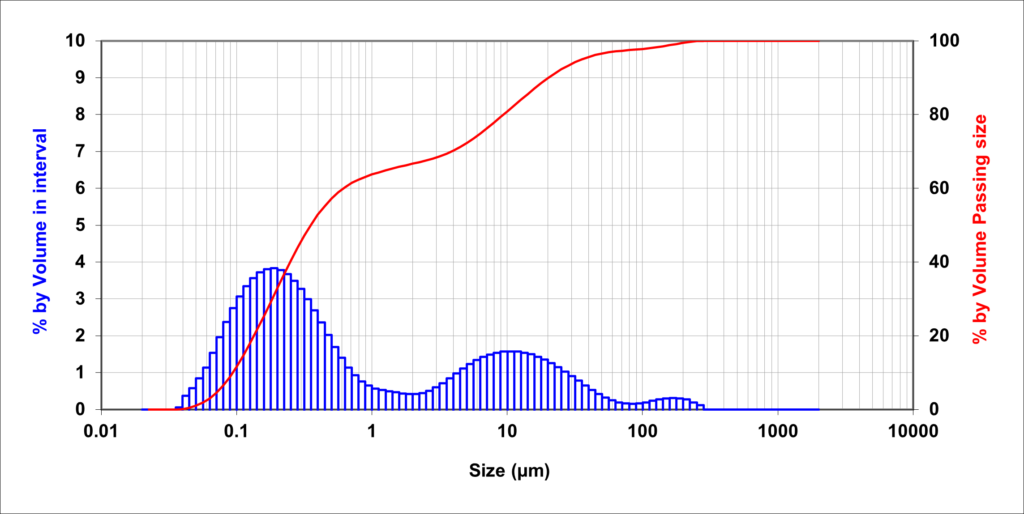
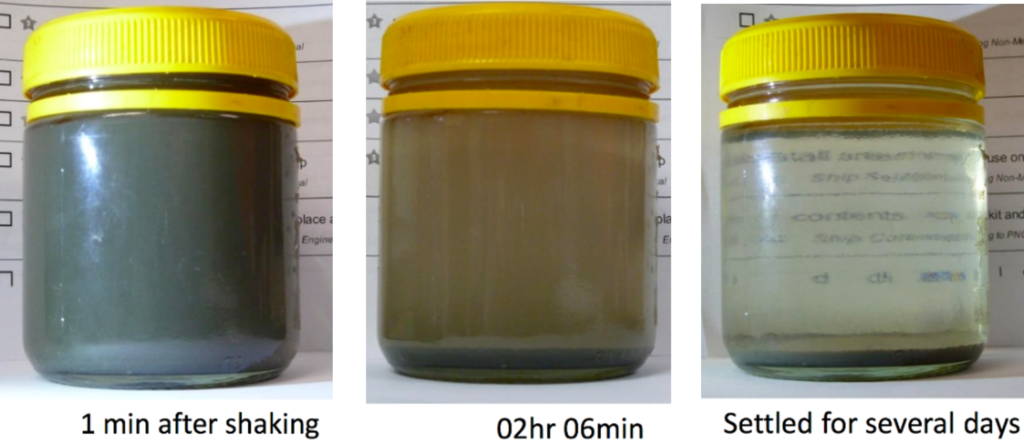
It is simple and cheap to test for colloids in your water. Colloids will effect the clarity of water – if the water is clear then there is not a high concentration of colloids. If your water is not clear then collect some of the water in a clear container and see how long any suspended solids take to settle out under gravity. The speed at which the particles settle out is related to their size. Any particles that don’t settle out after a day will be colloidal. If you do have colloids present, the next step would be to get a Particle Size Distribution to determine the range of different particles present in your water.
How can colloids be removed from water
Once you have confirmed that there are colloids in the water and that these particles need to be removed, there are a range of different technologies available:
- Coagulation filtration – adds a chemical that binds the colloids together, making them large enough to filter out using multimedia filters. Can be run either in batch or continuous mode and will need to be tailored for each specific water type.
- Polymeric ultrafiltration – membrane filtration technology similiar to nanofiltration and reverse osmosis but uses larger pores and lower pressures for filtration. Can require frequent chemical backwashes and air scours to stay in optimum working condition.
- Silicon Carbide ultrafiltration – ultrafiltration membranes built from ceramic materials. Abrasion resistant and can be run without the use of chemical. Small scale systems have higher CAPEX than coagulation filtration or polymeric ultrafiltration but larger scale systems are cost competitive

To discuss which technology is best to remove the colloidal particles in your water, contact Moerk Water today




